Our Class II medical device client needed to launch a new high-volume high-mix production line on a very short timeline. In addition, the line needed to meet strict FDA process guidelines. They lacked the resources and the experience in complex production line design, install, and validation to meet their tight deadline, while maintaining the quality standards required to receive FDA approval.

Our client needed to scale their production to meet market demand and wanted to take the opportunity to optimize the process as well. Key goals/challenges included:
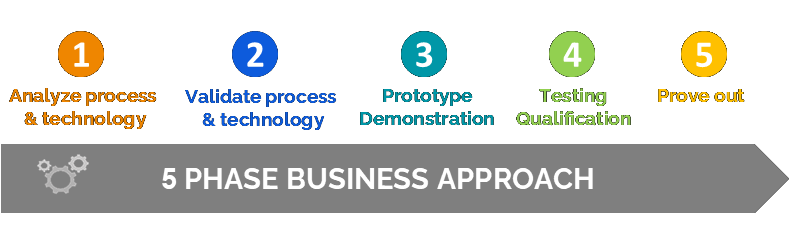
QFocus assembled a team with the necessary expertise and tackled this project using a proven 5-step business approach. After performing a thorough analysis of the existing plant process and technology, the team proposed an optimized design, validated the new process and did a prototype demonstration, before implementing the line, testing, and final qualification and approval.
The QFocus team worked in close collaboration with our client and external authorities to expedite approvals and meet the launch deadline. The resulting production line delivered:

Faster TTM

Higher Quality

Increased Margins